When: 15 - 17 May 2023
Where: TOPRA Office, 6th Floor, 3 Harbour Exchange, South Quay, London, E14 9GE
Day 1 start time: 09:00 BST | Day 3 end time: 15:15 BST
Please contact us directly for booking information and/or consider attending this course online.
Also available as a virtual course, please click here for the virtual registration option.
Course overview
This Masterclass covers:
- Different classes of biological products such as vaccines, monoclonal antibodies and gene therapy medicinal products
- Practical regulatory aspects of regulatory strategy for biological, biotechnology and advanced therapy products
- Regulatory Requirements for clinical trial applications and marketing applications
- The quality, non-clinical and clinical changes specific to biological products
- Implications of changes to the production of biological products and the concept of compatibility
- Case studies to apply the regulatory principles
The combination of lectures and case studies and workshops will assist in the achievement of the learning outcomes and enable the students to contextualise their understanding and knowledge.
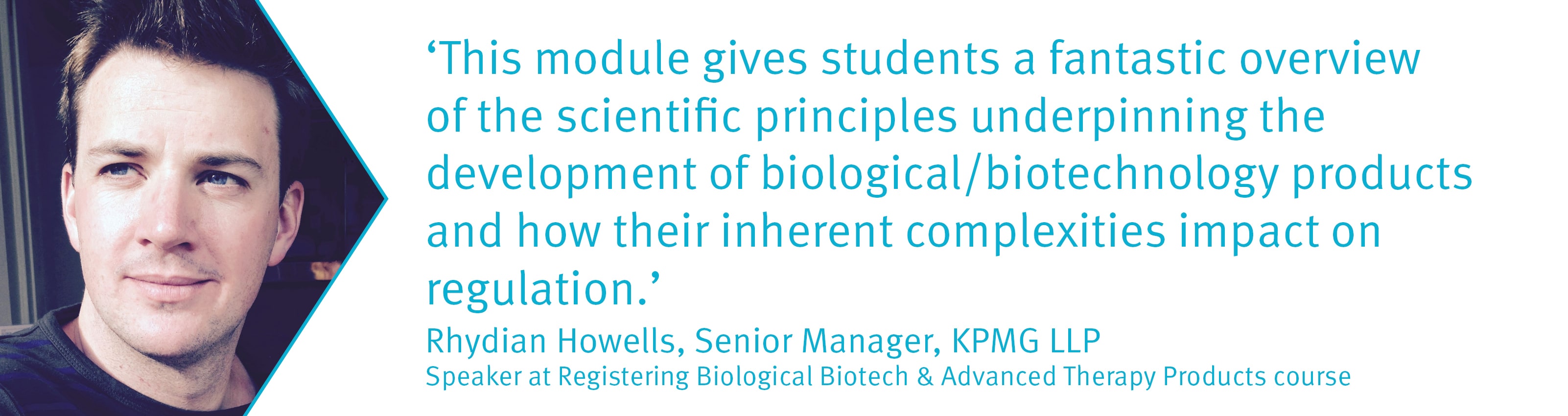
Benefits to delegates
This course will enable you to:
- Display a systematic understanding of knowledge and a critical awareness of the unique nature and strategies for development of biological, biotechnology and advanced therapy products
- Possess a comprehensive understanding of the regulatory requirements and associated documentation with the licencing of biological, biotechnology and advanced therapy products
- Demonstrate a conceptual understanding of the legal and pharmaceutical requirements that define the regulatory strategy for biological, biotechnology and advanced therapy products
- Deal with complex issues both systematically and creatively, make sound judgements in the absence of complete data, and communicate their conclusions clearly to specialist and non specialist audiences regarding biological, biotechnology and advanced therapy products
- Demonstrate the ability to critically analyse the legal documentation and regulatory considerations of biological, biotechnology and advanced therapy products
- Critically appraise and evaluate communications from regulatory bodies and research publications regarding biological, biotechnology and advanced therapy products
CPD: This course provides 15 CPD hours upon successful completion. To learn more about why Continuing Professional Development is important, visit our Lifelong Learning page.
Programme
You can view and download a programme here
Module Leader
- Rhydian Howells – Senior Manager, KPMG Life Sciences Regulatory Solutions Practice
Presenters
TOPRA Masterclasses are developed and delivered by a
faculty of expert speakers from relevant industry, agencies, notified bodies
and other stakeholders, and are validated by the University of Hertfordshire.
In addition to the module leader(s) listed above, speakers for this
course include:
Richard Keane - Sr Director, Head of Global Regulatory CMC Commercial Products, Biogen
Tara Hutton - Director, Biogen
Alison Wolfreys - Senior Director, UCB
Cecil Nick - Vice President, Parexel
Paula Urquhart - Senior Assay Development Scientist, Covance
Andrew Deavin - Director Vaccine Global Regulatory Affairs Asia Pacific and China, GSK Vaccines
Sergio Fracchia - Global regCMC Director Cell and Gene Therapy,Novartis
Zabin Younes - Scendea
Isabelle Cludts - MHRA
Suitable for
- Students of the TOPRA MSc programme
- Delegates from the regulatory affairs industry who wish to develop their knowledge of biological, biotech and advanced therapy products
- Delegates from allied industries who wish to have a comprehensive understanding of the subject
Pricing
Standard
- Student: £1,500 + VAT*
- Delegate: £1,700£ + VAT*
Discounted places
A limited number of discounted places are available at the rates below. Please note that discounted places are not valid for those enrolled on the MSc course.
Please email us at meetings@topra.org for a discount code before making your booking.
Discounted prices
- Those working for regulatory agencies, government agencies or academic institutions: £1,275 + VAT*
- Those working for charities, patient groups or in full-time education: £1,275 + VAT*
* VAT, if applicable, is charged at the rate of 20%
Terms and conditions
By booking a place on this course you are agreeing to the training terms and conditions.